The pH of coffee measures the degree of its acidity or basicity (alkalinity), and is expressed on a scale of 0 to 14. A pH of 7 is neutral, less than 7 is acidic, and 0 is the most acidic. More than 7 is basic or alkaline, with 14 the most basic. Coffee is acidic and has a pH between 4.8-6.30 with an average pH of 5. This variable pH range depends on the type of coffee beans you are using, grind size, roasting type, brewing time, and water temperature. The pH of coffee also depends on the type of coffee drink and the ingredients used in it.
The pH of coffee has no direct effect on the health and safety of the consumers. Yet it is one of the most important indicators of the quality and flavor profile of coffee. Coffee drinks which have a very low pH like black coffee, espresso, and americano have a bitter taste profile.
Coffee drinkers who have sensitive digestive systems tend to prefer coffee drinks having milk and cream. This is because milk and cream reduce the coffee acidity and prevent the stomach from gastric ulcers, heartburn, and gastroesophageal reflux disease.
What is the pH level of coffee?
The pH level of coffee varies between 4.8-6.30 within an average pH of 5. A pH of less than 7 means that coffee is acidic in nature. The variable range of pH is attributed to the vast ways of making a coffee drink.
What makes coffee acidic?
The organic acids and chlorogenic acids present in coffee makes it acidic. The organic acids making coffee acidic are: citric, acetic, formic, malic, quinic, pyruvic, succinic, fumaric, tartaric, glycolic, phosphoric, and lactic acid, as established by Sara E Yeager and colleagues from University of California Davis.
Chlorogenic acids are mainly responsible for the acidity and bitterness of coffee. Chlorogenic acids account for 5-10% of coffee beans whereas caffeine accounts for only 1-2%, as stated in the book ‘Coffee in Health and Disease Prevention’.
Acids make 11% mass of green coffee while 6% mass of roasted beans as found by a study published in the ‘Critical Reviews in Food Science and Nutrition’. The amount of acids in a cup of drinking coffee varies depending on bean species, roast level, extraction methods, and overall technique.
What factors affect coffee acidity?
The factors which affect coffee acidity are coffee bean type, grind size, roasting, brewing method, and water temperature as listed in the figure shown.
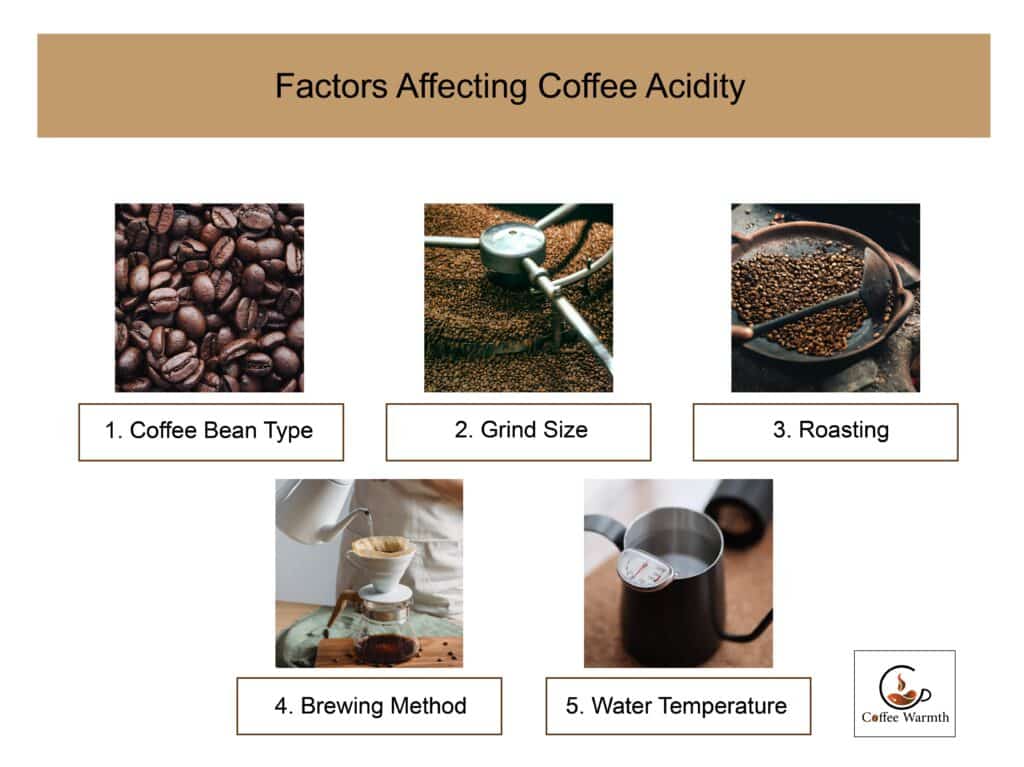
1. Coffee Bean Type
Coffee beans largely determine the acidic profile of a coffee drink. Origin of coffee beans determines the level of acidity because every soil has different characteristics. Arabica coffee beans are more acidic than Robusta beans as Robusta do not have citric and phosphoric acid. Arabica coffee beans even with a low pH level have a fruity, mild, and less bitter taste profile than Robusta.
Mané Alves, founder of Coffee Lab International, says that Kenyan coffee has higher concentration of malic acid while Colombian coffee has a larger proportion of citric acid.
Coffee beans grown at higher altitudes and lower temperatures are more acidic than the beans grown at lower altitudes and higher temperatures, as they ripen slowly, developing complex compounds. Coffee beans grown at lower temperatures are hard and have more acidity than the softer coffee beans.
The processing method, i.e., to remove coffee beans from coffee fruit, also determines the acidity of coffee. Out of the three natural processing methods, natural, washed, and honey, washed coffee is more acidic than the natural and honey method.
2. Grinding
Grind size of coffee beans affects coffee acidity. Coarser grind leads to a more acidic cup of coffee compared to fine coarse.
Coarse grind coffee increases acidity from 0.257 to 0.284 and fine grind decreases acidity from 0.31 to 0.17, according to a study in the Journal of the Korean Society of Food Science and Nutrition.
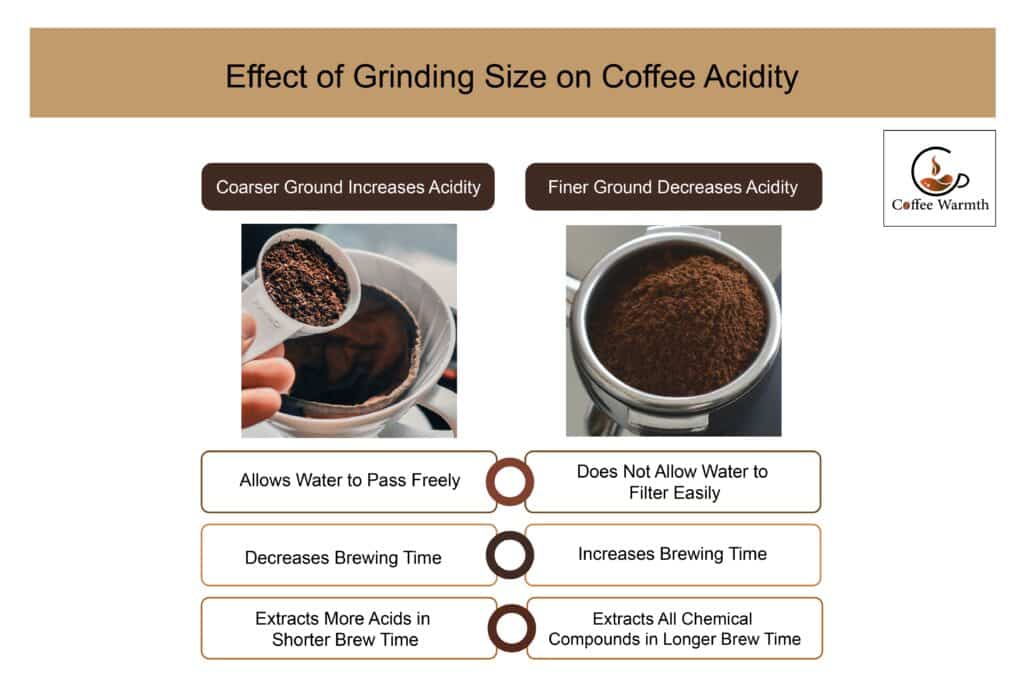
A coarser ground makes coffee more acidic as it allows water to pass freely through the ground decreasing the brewing time. Shorter brewing time means less extraction. At the start of the extraction phase, more acids are extracted from coffee beans as compared to other chemical compounds. So, with a shorter brewing time, mainly acids are extracted from the beans making the cup of coffee acidic.
A finer ground allows greater extraction as the surface area increases. However, a finer grind prolongs the brewing time because water has a hard time filtering through the finer grounds. It also allows the extraction of chemical compounds other than acids which makes coffee less acidic.
The graphic shown draws a comparison between coarser and finer ground size and its effect on acidity.
3. Roasting
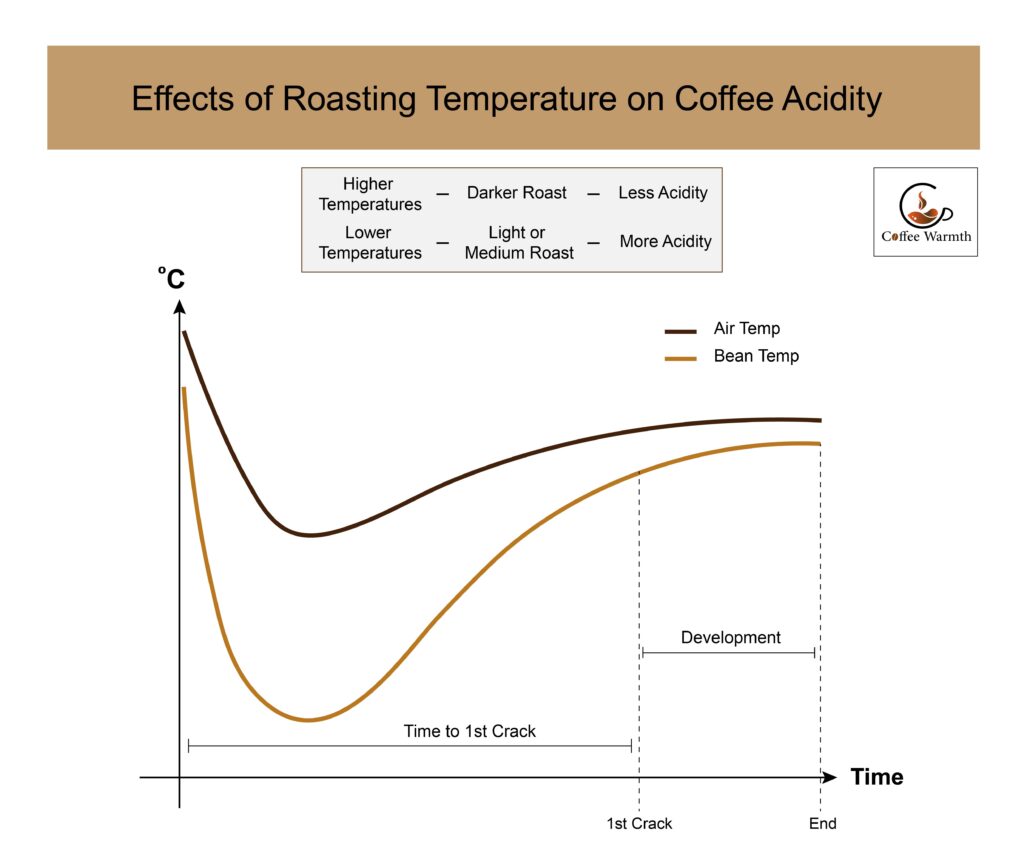
Roasting level affects the coffee acidity. Higher temperatures lead to a darker roast with less acidity, whereas lower temperatures make light or medium roasts with more acidity.
According to Verônica Belchior, Ph.D. in Food Science and a coffee quality educator, some acids decrease whereas some increase in concentration during the degradation of beans in the roasting process.
The two types of coffee acids; organic acids and chlorogenic acids increase or decrease in concentration during roasting. They are responsible for the sweet and bitter taste of coffee. Organic compounds correspond to the sweetness of coffee whereas chlorogenic acids give a bitter taste profile.
Bruno Danese, Head Barista and Roaster at Hoccino Coffee in Japan, says using high heat during roasting decreases acidity but too much heat can burn the beans. Rate of rise and temperature of the roasting machine should be high enough to achieve the first crack early. After the first crack, the temperature is increased slowly to form an S-wave. However, care needs to be taken because heating for too long after the first crack breaks the chlorogenic acids causing bitterness.
The line graph forming S-wave for both air and bean temperature is depicted below.
4. Brewing
Shorter brewing time leads to more acidic coffee due to less time for extracting all chemical compounds. Longer brewing time leads to less acidic but bitter coffee due to more extraction time.
The duration of extraction determines the compounds extracted from coffee. At the start of extraction, fruity and acidic compounds are extracted, whereas with prolonged extraction time sweet and bitter compounds are drawn out.
5. Water Temperature
There is contradictory evidence of the effect of water temperature on coffee acidity. Mané Alves, founder of Coffee Lab International, states hot brew generates a much higher acid concentration than cold brew.
Extraction is quicker at higher temperatures and certain compounds are extracted only in hot brew. At lower temperatures, brewing time increases which extracts all compounds giving a much fuller cup of coffee. John Behm, the founder and CEO of Behmor says that he gets more pronounced acidity in coffee at 95ºC/204ºF.
However, Dr. Niny Z. Rao from Thomas Jefferson University in his research refutes this claim. He concluded that cold brew has a lower pH and is more acidic compared to hot brew.
What are pH levels of different coffee drinks?
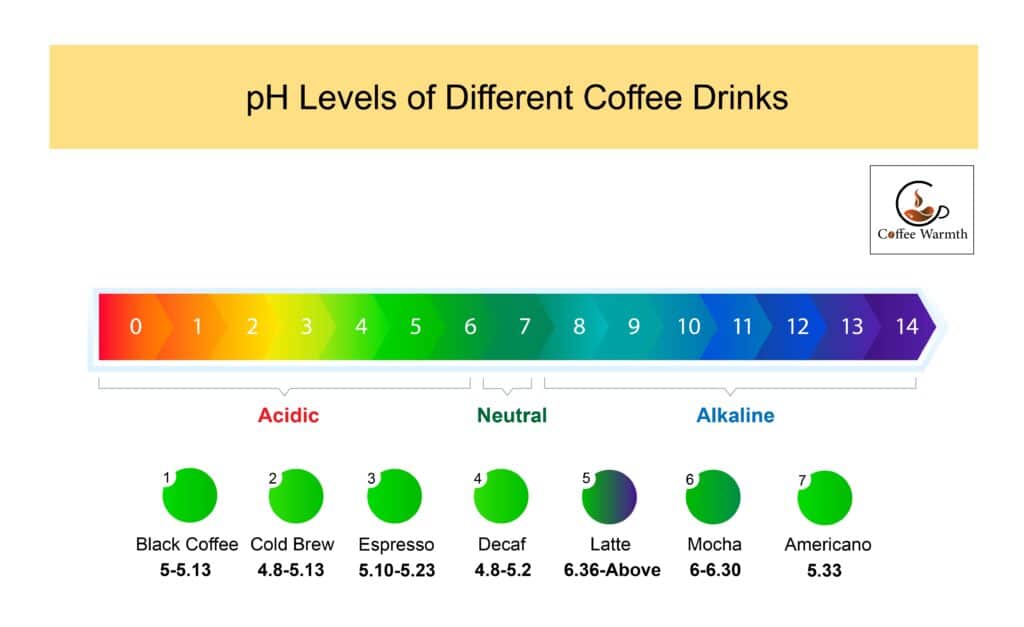
These are the pH levels of different coffee drinks.
1. Black Coffee – 5 – 5.13
2. Cold Brew – 4.8 – 5.13
3. Espresso – 5.10-5.23
4. Decaf – 4.8 – 5.2
5. Latte – 6.36 – above
6. Mocha – 6 – 6.30
7. Americano – 5.33
The pH levels of coffee drinks are variable depending on the coffee bean used, roast level, coffee to water ratio, and brewing methods.
The pH level of above-mentioned coffee drinks are shown in the diagram below.
1. Black Coffee
Black coffee is coffee without any additives like milk, sweeteners, or cream.
The pH of black coffee ranges from 5 to 5.13 which is mildly acidic. According to a study published in the Pakistan Journal of Medical and Health Sciences, the pH range of black coffee is 5.13±0.02.
Black coffee pH depends on types of coffee beans, roasting, and grinding method. The pH of black coffee is in the range of 5 because there are no additives like milk or cream in the coffee to increase the pH.
Drinking black coffee is safe but can aggravate digestive issues in people with sensitive digestive systems.
2. Cold Brew
Cold brew coffee is made by steeping coffee in cold or room temperature water for 12-24 hours.
The pH of cold brew coffee ranges from 4.8 to 5.13 as stated by Dr. Niny Z. Rao from Thomas Jefferson University. Dr. Rao’s research negates the popular claim that cold brew is less acidic than hot brew.
Cold brew high acidity is ignored due to its mellow and bold flavor profile. However, drinking cold brew regularly leads to low salivary pH and digestive issues.
3. Espresso
Espresso is a concentrated coffee drink made by passing hot water at high pressures through finely ground coffee.
The pH of espresso ranges from 5.10-5.23 according to Vladimir Ostry and colleagues from the National Institute of Public Health in Prague.
The pH of espresso is less than regular coffee because of high pressures, short contact time, and use of dark roast beans. Espresso pH varies depending on roast levels and brewing technique.
4. Decaf
Decaf coffee is a regular coffee which has all of its caffeine removed.
The pH of decaf coffee ranges from 4.8 to 5.2 and is slightly acidic. Decaf coffee lies in the same pH range as regular coffee.
Even though decaf coffee is equally acidic, it does not cause heartburn and acid reflux. The reason is that chemical compounds causing GERD symptoms are removed during the decaffeination process.
5. Latte
A latte is a milk coffee with one or two shots of espresso at the bottom, steamed milk in the middle, and a thin layer of frothed milk on the top.
The pH of latte ranges from 6.36-above depending on the pH of additives. Starbucks Caramel Latte has a pH of 6.36 close to the neutral 7 pH. Matcha Latte has a high pH due to the alkaline nature (7-9 pH level) of matcha green tea leaves.
A high pH range gives latte a sweet and creamy flavor profile. Drinking latte is easy for the stomach, reducing the incidence of acid reflux.
6. Mocha
Mocha or mocaccino, a variant of latte, is made of espresso, steamed milk, and chocolate.
The pH of Mocha ranges from 6-6.30 depending on the chocolate and type of milk used. Starbucks White Mocha with whole milk has 6.30 pH whereas pH with skim milk is 6.26.
Coffee and chocolate combined in Mocha lower the acidity but milk balances out the pH. The balanced pH makes Mocha a good low-acid coffee choice.
7. Americano
An Americano is just hot water and espresso served at a ratio of 1:2, 1:3, or 1:4.
The pH of Americano coffee ranges from 5.33 according to a study done by Vladimir Ostry and colleagues from the National Institute of Public Health in Prague.
Americano has a bold but less bitter taste than espresso. Since it is a diluted form of espresso, Americano is stomach friendly.
How does coffee acidity affect the stomach?
Coffee acidity affects the stomach by an increase in the secretion of gastric and hydrochloric acid. Caffeine in coffee secretes gastric acid by antagonizing adenosine.
The stomach pH is 2.0 or lower which makes the stomach environment acidic as stated by Ewe et al. in Advances in Virus Research. Increased gastric acid secretion further lowers the stomach pH leading to various gastrointestinal problems. These gastrointestinal problems are gastritis, peptic ulcers, poor digestion, flatulence, and gastro-esophageal reflux disease (GERD) in coffee consumers as illustrated in the figure below.
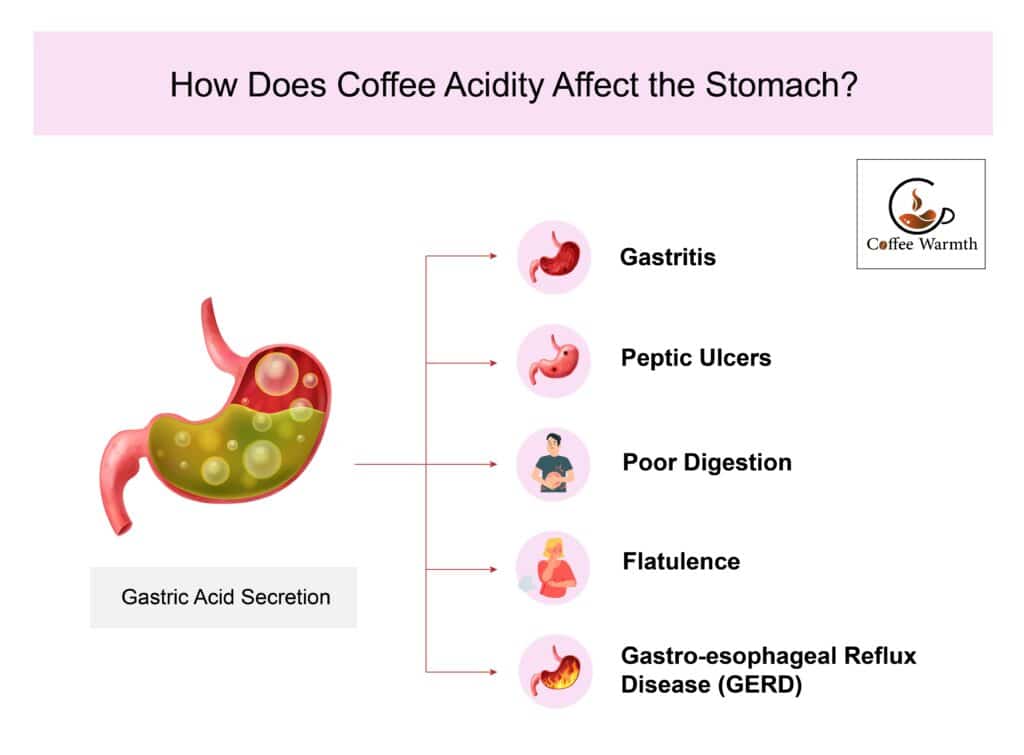
Increased release of gastric acid in those drinking coffee leads to gastric inflammation or gastritis in the long run. Drinking 3-4 cups of coffee daily is the leading cause of chronic gastritis as found in a study in the International Journal of Novel Research in Healthcare and Nursing. Gastric acid secretion and gastritis both predispose coffee drinkers to peptic ulcers, according to a study in The Lancet.
Dr. Boekema P.J. states that enhanced gastric acid secretion by drinking coffee promotes GERD symptoms. In a study published in Medical Science Monitor, Ji-Hao Xu et al conclude that coffee promotes release of gastric acid which in turn causes GERD symptoms like functional dyspepsia in regular consumers.
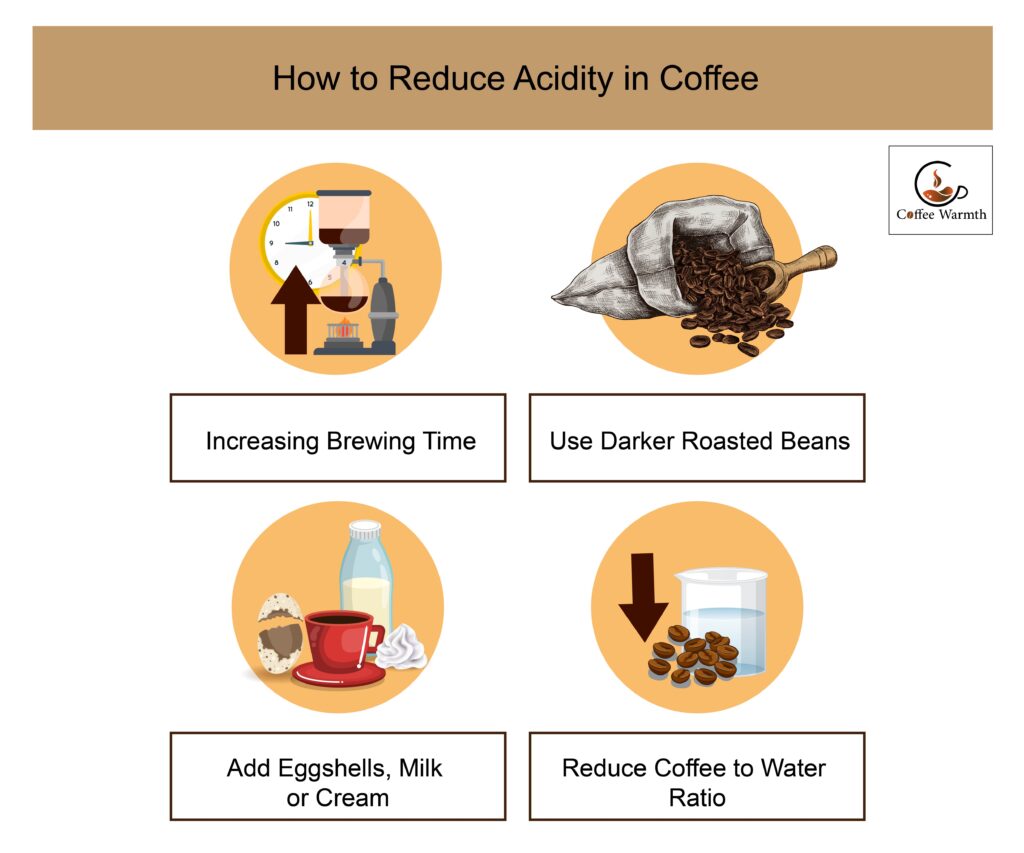
How to reduce acidity in coffee?
The ways to reduce acidity in coffee are by increasing the brewing time, using darker roasted beans, adding eggshells, milk or cream, and reducing coffee to water ratio.
You can reduce acidity in drinking coffee by increasing the brewing time. In this way, the water extracts all essential chemical compounds making a cup which is full of flavor but less acidic.
Use dark roasted beans which are naturally less acidic compared to other types. In addition with dark roasts, coffee acidity decreases when the beans are exposed to heat for a shorter time period.
One of the best ways to reduce acidity in coffee is by adding eggshells, milk or cream. Eggshells, milk or cream are rich in calcium which increases the pH of coffee by forming calcium carbonates.
Another simpler way to make coffee less acidic is by splashing some water. The ideal coffee to water ratio is one to two tablespoons or 15-30 grams of coffee to six ounces or 180 ml of water. Increasing the quantity of water would decrease the coffee acidity.
The image shown summarizes how acidity in coffee is reduced.
Does adding milk to coffee reduce its acidity?
Yes, adding milk to coffee reduces its acidity. Milk reduces acidity of coffee by two processes: combination of calcium with H+ ions and process of dilution.
The pH of milk is 6.5 to 6.9 depending on whether it is pasteurized, canned or dry milk, and pH of coffee is 4.85 to 5.13. Adding milk to coffee would dilute it as pH of coffee is lower than milk.
Calcium in milk reduces acidity of coffee by combining with H+ ions in the gastric and hydrochloric acid. Calcium and H+ ions form calcium hydroxide which reduces the concentration of H+ ions in the stomach and increases the pH.
Does adding creamer make coffee less acidic?
Yes, adding creamer to coffee reduces its acidity. Creamer, a powdered or liquid alternative to milk, reduces coffee acidity by dilution and combination of calcium with the H+ ions.
Creamer has a pH of 6.8 so it neutralizes the bitterness and acidity of coffee.
Combination of calcium in creamer with H+ ions works in the same way as milk. Calcium hydroxide is formed, reducing the acidity.
